|
It may be helpful!! :) Just go to the enumerated points if you don’t have time for the backstory :) Short backstory: as an early-stage researcher in the Allostery in Drug Discovery (ALLODD) Network within Marie Skłodowska-Curie Actions, I got lucky and honored by having an invitation by Dr. Zoe Cournia and Prof. Marco Cecchini (my Supervisor) to contribute, among many other authors, to the yet-to-be-published Review, which topic is connected with alchemical free energy calculations on certain systems, relevant to drug-design. While some final work is yet to be done before the publication, I wanted to share some of my insights on writing, which indeed was a novel experience for me. Although at the first sight the question of “writing a Review” may seem trivial for those who at the beginning of their scientific career already have got their hands on drafting a scientific article in a peer-reviewed journal or have done decent literature work in their Bachelor’s or Master’s Thesis, writing a professional Review may require some additional techniques and persistence. First, the difference between the typical introduction in a peer-reviewed article with your results and a Review is in how exhaustive you need to be to discuss a certain topic. Although in typical articles you certainly need to be well-informed of the current stage of scientific progress on your problem of interest, Reviews often discuss broader issues and may require a more complete literature analysis. Additionally, in your typical article, you may know the most recent advances and key articles through your Supervisor – but a Review could go beyond the scope of their direct expertise, and includes all the most recent advances – which could’ve been missed unless a thorough work to find them was done. Just imagine how many new scientific articles are out there each month. Thus, here are some tips for writing a scientific Review, at least what worked for me:
This is it! I hope that someone finds these pieces of advice helpful and not too obvious – but of course, know that this was only the perspective of someone for whom the Review preparation was a new experience, and who worked only on certain sections of the prepared publication.
1 Comment
Secondments are an essential part of our scientific journey as Early-Stage Researchers in the ALLODD ITN consortium. Essentially, they are research stays, carefully planned by our supervisors to both complement our Ph.D. projects and give us experience and expertise in different fields than ours. It is a time to gain new knowledge, acquire new skills and network with people from outside your line of research. It gives us a chance to see the bigger picture in science and discover the numerous possibilities in Allostery and Drug Discovery. In my case, my first secondment journey was from red-hot Barcelona to 10-degree Stockholm; from my familiar computational world to the not-so-familiar and complex reality of wet-lab experiments. Imagine a computational chemist with a background in chemical engineering having to express and purify their first protein. That was me 6 months ago. Very excited, a little scared, optimistic and ready to learn. My experimental journey began in the group of Dr. Galdeano, at the University of Barcelona, where I was taught how to grow bacteria, how to express protein and how to purify it. I am extremely thankful to my colleagues from Galdeano’s lab, Roger and Andrea, for their patience with me and my rusty experimental skills. With their guidance, soon my protein was ready for shipping to Karolinska Institute, to the Mass Spec lab of prof. Roman Zubarev. Together with the protein, I shipped myself to Sweden, leaving aside my computational studies and embracing my new challenge: the world of the MS techniques. For the next three months in the lab at Karolinska Institute, I learned something new every day- not only about the MS experiments but also about the experimental work style. During the first few weeks, together with my ESR colleague, Bohdana Sokolova, I was trained on how to prepare HDX-MS samples, how to handle MS instruments and how to analyze MS data. Along with the HDX-MS methodology, I also learned experimental design from A to Z, planning based on instruments’ availability and lab time organization. It was a time of everyday lessons; some were easy and some were hard. The hardest lesson for me was accepting that experiments don’t always work out. My optimism wavered when my first HDX tests did not give the expected results. My hopes diminished as we stumbled upon drawback after drawback. Sometimes the established experimental technique works perfectly for your project, but other times it needs parameters’ optimization and even further development to meet the needs of the scientist. Even then, the method may not provide the results it did in others’ research and it may even lead to a dead end. But I learned this is okay! I understood failure is a natural part of the experimental process and it is essential to be aware of the opportunities and the limitations of each technique. With every method mastered you also gain experience on how and when to use it and this lesson will always stay with me. In the line of learning and acquiring new skills, my experimental journey did not end with the HDX-MS technique. With Dr. Gaetani’s guidance, I set off to gain experience in the Proteome Integral Solubility Alteration (PISA) assay, developed in Zubarev’s lab. Now imagine a computational chemist in a cell lab- again, that was me. It was not easy at first- everything about the cell lab was new to me; every skill, every piece of information sounded foreign. And, of course, I did make some mistakes. But each mistake turned into a lesson. Through trial and error, through practice and patience, in the last week of my secondment, I felt like a well-oiled machine with tissue culturing and cell treatment. And it was likewise with every other part of the 5-day PISA procedure I had to master to be able to perform the designed experiments. It was an amazing experience- careful planning each day according to the instruments’ availability; getting familiar with many different practices; working on a tight schedule with many tasks. Some days nothing was working, some days went smoothly and exactly as planned- a true experimental rollercoaster. And just like that, I conducted the planned PISA experiments and my secondment journey came to an end. I felt a huge sense of fulfillment- the three months experimental stay in Stockholm has turned me, a computer-based young scientist, into an experimentalist. I wouldn’t say that now I am an expert on MS-based methods; but I am sure the skills and experience I got from Karolinska Institute will help me through the next steps of my career and I am grateful for this opportunity to everyone involved. Now that I am back to my computational studies, I am looking forward to my next secondment and to learning more about the experimental part of drug discovery. And I would recommend to every computational scientist that hasn’t been in the lab for a long time- go out of your comfort zone, make the journey and experience research from the other side. It is worth the rollercoaster! And some unforgettable moments from the journey (whenever I remembered to take a photo)! Here we are, it's been a year since I started the ALLODD journey… and what a year!
In January 2022 I settled down in Germany, which administratively was not so smooth. After a few days, I officially started in the lab at the biggest site and also headquarters of Merck. It is like a small town (we are more than 12´000 people working there). Despite being the only non-German speaker in the lab, any single person speaks English, which made my first months a lot easier that I would expect by being somewhere you don’t speak the language. Actually, I learned German at school a few years ago and let me say it is not like riding a bike you will forget if you do not practice! I hope, by the end of the project, to be able to speak fluently in German. This is also why I applied to learn more scientifically and personally. As the first weeks and months go by, I was learning more about the different machines in the lab and their corresponding assays, as well as going deeper in the literature. September was the opportunity to go to Barcelona in Prof. Dr. Curutchet lab as my first secondment. Moving from Darmstadt to Barcelona was definitely a weather improvement. Even though the surroundings were not new for me, I discovered there a new scientific world: the computational world. From opening a terminal to running my first molecular dynamic simulation, it was a very broad journey. This was also my first experience working at the University, as I did all my internships in private companies. I enjoyed my stay in the group, I am very thankful for the time they devoted to me, to explain and help me or to discover new restaurants. Now back in Germany, it´s time for me to be reunited with the lab experiments and my cells: Saccharomyces cerevisiae. Yes, those are the same cells used in bakery or brewery (I had to adapt to the German culture 😉). Being part of an ITN also means business-travel, during this first year, we met in-person twice with the wonderful people of the ALLODD network. Vienna, Barcelona and soon Strasbourg and Budapest, we emphasized the words network and partnerships! The best is yet to come! When my turn came to write a blog post, most of my colleagues have already had a chance to talk about their experience as fresh PhDs. Joining a high-level research group, meeting renowned scientists, winter schools, conferences, exchanges abroad – many things that one could only dream of in undergrad are most frequently mentioned in the reflections on first months of doctorate studies. But alas, from my own experience and discussions with other PhD students I dare say that the feeling that often hides behind this refined façade is frustration. Questions like ‘where is this going?’ and ‘am I doing enough?’ used to pop up in my head all the time adding to the general state of confusion as a newly-started PhD student. Having successfully survived 5 months of my new role, I would like to leave some tips for those who are just starting their journey and myself on how to get through first months of PhD studies.
‘Accept your destiny’ First of all, it is normal to feel confused. We will skip first four stages of Bart Simpson’s scheme right to the last one – acceptance. Embrace the idea that you will not be perfect on this path, you might not see the global idea behind the project, you might not understand everything, you might make mistakes, and it is completely okay. Regardless of what you have been doing before, no one is fully prepared to be a PhD student. Once you have accepted your fate, you can move on to the next step. ‘What can I do to make this journey as smooth as possible?’ Start learning about your project asap. PhD is a combination of studies, research work and – most importantly – managing a project, and you cannot lead this project without knowing the direction where you are going. So read the literature, discuss it with your supervisors, make sure to have a clear vision of everything that is supposed to happen during these 3 (or 4) years. ‘Use the opportunities’ Consider your time as a graduate student a mutually beneficial agreement between you and the scientific community: you provide an in-depth research on a certain topic and in exchange get an opportunity to grow personally and professionally. PhD studies provide space to improve a large variety of skills. Always wanted to start writing? Launch a personal or write articles for biotech media. Interested in trying yourself as a mentor? Volunteer to supervise an undergraduate student or work as a TA in a course. Organizing conferences, starting a pop-science podcast, exploring bioenterpreneurship – there are countless possibilities out there waiting for you to uncover them! Think about your passions and try to find ways to express them as a part of your studies from day one, it will certainly bring a splash of color into your routine work (and enrich your CV😉). ‘Network’ This is your unique chance to meet like-minded people ready to change the world. So put yourself out there, attend workshops, conferences, learn about things that others are doing (even if they are not directly applicable to your field or rather especially if they are not directly applicable to your field). ‘Healthy balance’ Last but not least, remember that research is just one part of your life. As important as your work is for you, it is crucial to find that ‘equilibrium state’ where you could have space for healthy sleep, sport, hobbies and private life. PhD is a marathon and not a sprint, so make sure you have enough energy to make it to the finish line! It has been already 6 months since I arrived to the lively, ancient and history filled city of Urbino. This is not only my first time leaving my country behind, but it is also my first time living by myself, so jumping into this project has definitely proved to be a real challenge.
A time comes for all of us to become an independent adult and deal with all kinds of new (and sometimes scary, of course) issues and situations. And if that you add the fact that your known environment, people and even language change it can become a challenging situation to be in. The biggest tip I can give to anyone is don’t be afraid to ask. And I mean in any situation you may find yourself in. Especially if you are a new to a city, of course you are not expected to know where things are or how anything works there. Being an introverted person myself, I usually struggled trying to approach people and asking the most basic stuff. However, something I learned is that people want to help. Even if you don’t share a common language, there is always a way to communicate and let them know what you need. This tip basically applies to most of the situations you can get yourself into:
When moving from Barcelona (1.6 Mil Population) to Urbino (14.4 k Population) I already expected a big change, not only in the city itself but also in the daily life. And that is exactly what I got. I now live in a quiet and beautiful walled city, surrounded by a landscape I never imagined. The flow of information from protein sequence over structure to physiological function has been boggling physicists’, chemists’, and biologists’ minds for over half a century. First postulated by Christian Anfinsen in the 1970s, the ‘thermodynamic hypothesis’ describes a unique relationship in which the amino acid sequence of a protein should be sufficient to determine its structure [1]. Yet, it was during the same time when Cyrus Levinthal famously noted that, theoretically, it would take longer than the age of the universe for a typical protein to sample all possible conformations in order to reach its correct fold [2]. Opening one of the biggest challenges in biology, advances in computational methods, as well as the drastic increase in experimental structures and sequences being published, just recently narrowed down the ‘sequence-structure gap’ that Anfinsen and Levinthal opened. Culminating in AlphaFold 2 [3], sequence-based structure prediction has reached astonishing accuracy, representing a huge leap forward in biology and also in drug discovery.
Is it that simple? While it is true that structure models represent a great starting point for many research endeavors, the same scientific advances that brought us AlphaFold revealed gaps in the sequence-structure-function paradigm. It is now common knowledge that proteins are not static at all, as suggested by models obtained from predictions or crystallographic structures, but highly dynamic. These dynamics range from bond vibrations to large conformational changes, come in networks, and often modulate protein function – a phenomenon collectively described as allostery [4]. But what is the relationship between sequence and the structural dynamics that govern function? If the sequence-structure-function paradigm holds true, these dynamics should be imprinted in the sequence and should evolve alongside the often highly conserved active site of a protein. More specifically, to maintain biological robustness, a network of energetically coupled residues should translate into a joint evolutionary constraint between each participating residue - they co-evolve or co-mutate [5]. Besides positional conservation, information about co-evolution is contained in multiple sequence alignments (MSAs) of homologous sequences, as these reflect the ‘evolutionary history’ of a protein family. By applying statistical models to MSAs, the interdependency of the variability of each sequence position, the ‘co-evolutionary coupling’, can be obtained and can be interpreted as direct or indirect physical connectivity between residues. A plethora of methods that build on this principle have been developed and succeeded in deciphering residue-residue couplings for structure prediction and identification of functional domains for ligand binding or allosteric regulation [6]. However, experimental validation of dynamic co-evolving networks that modulate protein function is challenging. In particular, when these dynamics take place in the absence of global structural changes. In a recent study, Torgeson et al. [7] combined co-evolutionary analysis and nuclear magnetic resonance (NMR) spectroscopy to identify previously undescribed dynamic networks of the protein tyrosine phosphatase (PTP) PTP1B. PTP1B’s structure, dynamics and function, as well as that of its homologs have been rigorously characterized, making it an ideal system to study the impact of co-evolution on functional dynamics. Torgeson et al. applied so called pseudolikelihood maximization direct coupling analysis (plmDCA) [8] to an MSA of PTP1B homologs to derive co-evolutionary couplings and then used a spectral clustering approach to split the structure into strongly coupled co-evolving domains, referred to as evolutionary domains (EDs) [9]. Supporting the idea of co-evolutionary analysis to identify functionally critical residue groups, four of the obtained EDs have been previously verified experimentally in PTP1B. However, further clustering revealed additional, yet uncharacterized subdomains. More than 16 Å from away from the active side, one of these subdomains contains an extended hydrophobic pocket that appeared to be, other than the already characterized EDs, contiguous in space rather than in sequence. Selectively mutating central positions in the domain, either independently or as triple mutants, reduced thermal stability in all cases, but increased the catalytic turnover rate (kcat) of the enzyme by more than 2-fold. Strikingly, structural analysis by 2D-[1H,15N] and 2D-[1H,13C] transverse relaxation optimized spectroscopy (TROSY) NMR and X-ray crystallography revealed no large conformational changes due to the mutations and previously described allosteric pathways of PTP1B remained unchanged. In the absence of global structural change, Torgesen et al. reasoned that an increase in kcat could be driven by side-chain dynamics in the µs – ms time range – a relationship that has been previously shown to govern the catalytic cycle of PTP1B [10]. To proof this hypothesis, they conducted so called constant time 13C Carr-Purcell Meiboom-Gill (ct-CPMG) relaxation dispersion experiments that allow for measurements of side-chain dynamics and extraction of a model that describes the conformational exchange between two populations A and B with the exchange rate kex. In measuring relaxation dispersion in the absence and the presence of a substrate-mimicking inhibitor, they could show that under conditions of catalysis (i.e., when the inhibitor is bound) the overall fast dynamics of the free mutated PTP1B are quenched and that residues cluster into three groups based on their kex values. Although these three groups were also identified for the wildtype, the exchange rates of the groups differed. Remarkably, one group contained many residues of the distal co-evolutionary subdomain described above and showed ~2-fold increase in kex from wildtype to mutant PTP1B – an increase that mirrors the 2-fold increase in kcat that was observed in enzymatic assays. The correlation of catalysis and side-chain dynamics in this group of residues was further supported by the resemblance between kcat and the fitted unidirectional exchange rate kAB. Most importantly, this also reflects the reciprocity of this regulatory pathway, because changes in the active site, e.g., binding of a substrate, changed the dynamics in the subdomain, while perturbations in the subdomain due to mutations influenced catalytic activity. But what is the purpose of this regulatory subdomain? While highly conserved residues of the hydrophobic subdomain support the N-terminal portion of an α-helix, which directly connects to the catalytic loop, less conserved residues flank the C-terminal portion of that α-helix. Accordingly, sequence variations in this less conserved part could enable fine-tuning of the kinetic properties of PTPs without perturbing specificity of the active site or the allosteric regulation. In mechanistically proving the relationship between a co-evolving non-catalytic subdomain and its impact on enzymatic catalysis Torgesen et al.’s study provides a good view on how functional dynamics can leave a footprint in protein sequences throughout evolution. The combination of co-evolutionary analysis with NMR-based analysis of side-chain dynamics proved to be critical in dissecting the regulatory network, otherwise invisible in static X-ray structures. Although this is just one of the few examples in which the energetic connectivity that underlies residue co-evolution was studied experimentally in detail, the study demonstrates that sequence analysis can be instrumental in mechanistic studies of protein dynamics. Finally, and most importantly, by showing that functional dynamics are indeed encoded in sequence, the study supports addition of dynamics as a missing part to the sequence-structure-function paradigm. References 1. Anfinsen, C. B. Principles that Govern the Folding of Protein Chains. Science 181, 223–230 (1973). 2. Levinthal, C. Are there pathways for protein folding? J Chim Phys 65, 44–45 (1968). 3. Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). 4. Wodak, S. J. et al. Allostery in Its Many Disguises: From Theory to Applications. Structure 27, 566–578 (2019). 5. Göbel, U., Sander, C., Schneider, R. & Valencia, A. Correlated mutations and residue contacts in proteins. Proteins Struct Funct Bioinform 18, 309–317 (1994). 6. Juan, D. de, Pazos, F. & Valencia, A. Emerging methods in protein co-evolution. Nat Rev Genet 14, 249–261 (2013). 7. Torgeson, K. R. et al. Conserved conformational dynamics determine enzyme activity. Sci Adv 8, eabo5546 (2022). 8. Ekeberg, M., Lövkvist, C., Lan, Y., Weigt, M. & Aurell, E. Improved contact prediction in proteins: Using pseudolikelihoods to infer Potts models. Phys Rev E 87, 012707 (2013). 9. Granata, D., Ponzoni, L., Micheletti, C. & Carnevale, V. Patterns of coevolving amino acids unveil structural and dynamical domains. Proc National Acad Sci 114, E10612–E10621 (2017). 10. Torgeson, K. R., Clarkson, M. W., Kumar, G. S., Page, R. & Peti, W. Cooperative dynamics across distinct structural elements regulate PTP1B activity. J Biol Chem 295, 13829–13837 (2020). 20th Sep 2022 - Secondment number one, what a joy it has begun, Budapest - what a great city, Day or night, it always looks pretty. Starting in the "ttk CompChem" team, I soon setup my first parameter screen, The objective; mapping allosteric GPCR binding, considering chemistry, energetics, and membrane for the finding. The project also took me virtually oversea, starting new collaborations with Boston University. Beyond - I got insights into QM/MM calculation, which are important for covalent drug creation. I signed up for a half-marathon, and visited the lake Balaton. Hungarian culture - opera, music, and dance are a treat, and the Kakaóscsiga is now my favourite sweet. 21st Oct 2022 - Today is already my farewell, one month went quickly, as I can tell. Thank you for the beautiful time. Being part of ALLODD is truly sublime. Saturday, 6 am. Berlin’s central station is deserted (apart from a few Oktoberfest aficionados on their way to Munich) when I board the train that would take me to Budapest. One year into my PhD, time has come to depart for my first secondment. After having moved to Berlin, struggled to find an apartment and settled in a new lab, the script repeats itself. Luckily, one gets more and more used to relocations over time. Packing and saying farewells become a routine and – let’s be honest – the 2.5 months that I will spend in Budapest are a foreseeable period of time. Based on my first impressions, Budapest is a bustling city, enchanting with its mixture of crumbling Art Nouveau buildings, modern skyscrapers and panel buildings (Panelház) that reflect its moved history. It seems to be a wonderful place to live, and probably most important for me right now, to do science! I was provided a very warm welcome by my host lab, the Medicinal Chemistry Research Group at the Research Center for Natural Sciences and am now looking forward to getting my chemistry running here.
When I was younger, I assumed that one would need to become a world-renowned music star or at least a diplomate to travel the world for work and live in different countries. Since I cannot sing and my French is rudimentary, I ruled out these career options early on. The thought that a career in science would get me there had never occurred to me. University studies, conferences, secondments – the list of occasions for travel and stays abroad as a scientist (as a chemist in my case) is long. The basic principles of chemistry and physics apply across borders; the stereoselectivity of an SN2 reaction is the same in Canada and Cameroon, and dichloromethane will form the lower layer in a liquid-liquid extraction with water anywhere around the globe. The language of chemical formulas and mathematical expressions is beautifully universal. Consequently, work in science is predestined for international exchange. I like to think of science as a connective link between people and cultures but of course, it is a valid question why this cross-country mobility is important and how society in general benefits from it. Taking a broader view on the meaningfulness of cross-border scientific exchange, I am convinced that it does not only accelerate scientific progress but likewise enhances multilateral collaboration to tackle the most pressing challenges of our time. It makes us aware that people anywhere around the globe are asking the same scientific questions and work hard to answer them. Collaboration in science can open hearts and doors to initiate partnerships in entirely different fields. The experience abroad gives us the possibility and at the same time responsibility to advocate for intercultural communication and tolerance towards different viewpoints, promoting diversity in our communities. On a personal level, I realize that each stay abroad confronts me with different opinions and ways of living, making me more approachable and understanding. I have become more resilient and self-confident by overcoming unanticipated challenges. Over time, I have learned most problems can actually be solved, often with the help of others. Moving to different countries has sensitized me for things that are not going well in places where I have lived before and, on the other hand, made me appreciate even more those things that were working out smoothly. Of course, personal development is one thing, but I am also convinced that each stay abroad makes me improve on a scientific level. During the current secondment in Budapest, for instance, I am re-learning lab-techniques that I am rarely using in Berlin (simply because I was not aware of their applicability to certain problems) but that might save me a lot of time during my daily work. I am also learning how to perform experiments, types of chemical reactions and work procedures that are entirely new to me but standard in my Hungarian host lab. Taking this knowledge back to Berlin and sharing it with my colleagues there, will benefit all of us. Finally, working and discussing with my new colleagues, who have different backgrounds and experiences, makes me see certain things, e.g. steps of a procedure, from a different perspective. This deepens my understanding of the respective processes and makes me question routine operations. Of course, I am very much hoping that this process of knowledge sharing is not one-way but mutual to make this experience most rewarding for everyone involved. Researcher’s Night is a vibrant festival of research, technology and innovation that takes place every year on the last Friday of September in many cities across the European continent. In Athens, it is hosted by one of the oldest and most prominent university foundations of the country, the National and Technical University of Athens, and it attracts numerous professionals from the educational field -teachers, professors, officials from the Ministry of Education- and, mostly, school and university students: children, teenagers and young adults aged 4 to 25. Given its non-specialized audience that mainly consists of younger individuals, the central concept of this particular event is for researchers to share their work in a more creative and original manner in order to give visitors the opportunity to gain a more hands-on perspective of their scientific activities. ALLODD successfully participated in this year's Research's Night in Athens, giving the opportunity to more than 250 interested individuals of all ages to get to know ALLODD Network, its objectives and actions. Being the first ITN to explore the concept of allostery in drug discovery, ALLODD aims to train the next generation of young scientists to exploit this concept and set the foundation for the emergence of the field. Cournia Lab was there in Researcher's Night in Athens, to communicate the science behind ALLODD to the general public. Such actions are of great importance and a major goal of the network, in order for more people to get familiar with the role of allostery in drug discovery. You can find the material of the event by clicking in the button below.
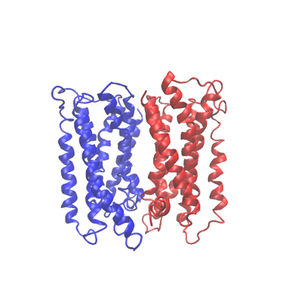
GPCRs are a large family of transmembrane proteins representing a renowned target in drug discovery. Classical GPCR drugs are simply developed by targeting their orthosteric binding sites, yielding compounds that either activate or inactivate the protein. The main problem with this approach is that these sites are highly conserved among GPCR subfamilies, and this causes poor selectivity and possible side effects. For this reason, in recent times the development of allosteric drugs, targeting GPCRs at sites that are different from the orthosteric binding sites, is getting increasingly relevant. The existence of such drugs has opened up the way for new therapeutic approaches and enriched the possible ways to modulate the functions of GPCRs1. Many studies have underlined the importance of allosterism in the context of GPCR dimerization or higher-order oligomerization in the control of the physiological responses they modulate. Indeed, for many years, GPCRs have been studied as single functional units (i.e., monomers)1. However, recent evidence suggests that GPCRs can also work as higher-order oligomers constituted by equal (homo) or different (hetero) monomers. In the case of oligomers, allosterism has a dual nature. On the one hand, a ligand alters the conformation of one monomer which then binds and modulates the configuration of the interacting receptor. On the other, the monomer itself can be considered as the allosteric modulator altering the conformation of the associated receptor, modulating its downstream efficacy and ligand affinity.
Therefore, GPCR oligomers have the potential to markedly expand the diversity and specificity of G protein-coupled receptor signaling, particularly in neural cells, where a few key receptors have been implicated in many neurological and psychiatric disorders, including addiction. Several approaches have been designed to develop new drugs specifically targeting GPCR dimers. One possible way is to design a so-called bivalent ligand 2, a molecule composed of two pharmacophores that span the length of the dimer allowing it to dock at both ligand binding sites simultaneously. An example of this was reported by Gmeiner et al. in 20163 with a study focused on targeting the D2R- NTS1R heteromer via three different bivalent ligands. In addition to this, it is also possible to develop bitopic ligands that bind the allosteric site of a monomer and simultaneously modulate the functions of the other associated functional unit. This is the case for instance of the SB269652 allosteric modulator of the Dopamine D2 dimer4.
|
- Home
- People
- Research
-
Training
-
Training Events
>
- 1st Workshop and PhD Induction Course
- 1st Training School & Networking Meeting
- Allostery in Drug Discovery Awareness Event and Symposium
- 2nd Training School & Networking Meeting
- IPR Training for Researchers & ESR Presentations on the Progress of their Research
- 3rd Training School & Networking Meeting
- European Conference in Allostery in Drug Discovery
- Secondments
- ALLODD Webinars
- Courses & Lectures
- Journal Club
-
Training Events
>
- Dissemination
- Events
- Newsroom
- Job Openings
- Blog
- Contact




 RSS Feed
RSS Feed
